Research: General
Hydration Water
Even though water appears uniform on a macroscopic scale, it behaves quite differently on the nanoscale. If we zoom in to the first few layers of water molecules near the surface of a protein or polymer (in fact, near any macromolecule or macromolecular assembly), we see that hydrogen bonds break and individual water molecules move at rates anywhere from two times slower to hundreds of times slower than those in the bulk liquid. We know that these differently behaved “hydration waters” are key to determining interactions.
The future of physical and analytical chemistry will center on the fact that, while tools have been developed for characterizing static structures, we still need techniques that can study dynamic arrangements of molecules and, most urgently, the arrangement of water molecules.
Comprehensive methods for mapping out the structure of the hydration layer do not currently exist. The future of chemistry, broadly, depends on techniques that will map out the structure of the dynamic solvent that surrounds macromolecules. The resulting insight will improve our ability to design drugs and synthetic materials. In particular, by developing technologies that can map out the dynamic structure of hydration water, we unlock an ability to design drugs that bind to proteins currently deemed “undruggable,” we can understand the energetics that cause signaling proteins to bind to their partners, and design polymer materials that conduct water in unique ways.
Most chemists and biologists recognize one very simple example of hydration water: the layer of water surrounding oil molecules that drive the separation of water and oil. But the more general case, especially for macromolecules that present a surface with variable properties to the solvent, is far more interesting and far less understood.
How do Proteins Alter Nearby Solvent?
Proteins are the molecular motors that drive all life, and most drugs are designed to interact with proteins. It is increasingly clear that water molecules at the surface of proteins control their structure and function; while researchers have developed methods that can study the structure of proteins, there are no accessible methods to spatially map the hydration water. We develop and apply magnetic-resonance-based spectroscopic methods for routine, robust, and accessible measurements of the properties of these key hydration water molecules.
A fundamental example
As a fundamental example, consider lipid bilayers – structures that make up cell membranes. Dr. Franck and his colleagues previously developed a specialized technique to characterize water near the bilayer surfaces. Our technique – called Overhauser Effect Dynamic Nuclear Polarization (ODNP)1,2 – tracks the motion of the water nuclei, and allows us to zoom in on the “hydration water” near the surface of the lipid bilayer (figure below). It allowed them to see that the hydration water moves about 5 times slower than bulk water3. Furthermore, by dramatically changing the characteristics of the bulk solution, we learned that an order of magnitude change in the viscosity barely affects the diffusion in the hydration layer3. They have also implemented this technique in systems with membrane proteins4, DNA5, and large protein folding chaperones6. The significant variations in the properties of the hydration water play in important role in how these surfaces interact in nature.
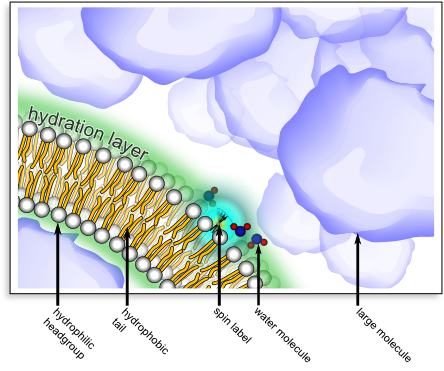
This image illustrates ODNP of water in the hydration layer of a lipid bilayer. This is one example of many studies that are possible with ODNP. A lipid bilayer (the basis for cellular membranes) consists of a self-organized set of molecules with hydrophilic headgroups and hydrophobic tails (see labels above). One can place a spin label (typically a nitroxide group) at a particular location near the surface of the lipid bilayer (via chemical synthesis). A combination of electron- and nuclear-spin resonance (i.e. ODNP) can selectively interrogate the motion of water molecules at a specific location – here inside the hydration layer of the lipid bilayer. For example, employing the setup shown above, we were able to verify that the motion of water molecules within the hydration layer was not sensitive to the presence of a high concentration of large molecules that are not chemically disposed to disrupt the hydration layer. This was even true when the large molecules significantly altered the viscosity of the bulk solution3.
Current Research:
In the Franck lab, we currently push the limits of the ODNP technique to retrieve new and more detailed information about hydration water. Towards this end, we draw on expertise in spectrometer design7–9 and advanced magnetic resonance methods10,11. We have already developed a cogent explanation of the ODNP technique2, as well as a new, powerful, and general scheme for visualizing and processing magnetic resonance data12. We are investigating the water on the insides of reverse micelles, which provide controllable pockets of water, as well as mapping out more subtle details in the water along the surface of proteins, both globular signaling proteins, as well as trans-membrane proteins.
References (Selected Publications)
Link to Complete List of Publications
[1. ]{.csl-left-margin}[Franck JM, Pavlova A, Scott JA, Han S. “[Quantitative cw Overhauser effect dynamic nuclear polarization for the analysis of local water dynamics]{.nocase}.” Prog Nucl Magn Reson Spectrosc. Elsevier B.V.; 2013 Oct;74:33–56. PMID: 24083461 doi:10.1016/j.pnmrs.2013.06.001]{.csl-right-inline}
[2. ]{.csl-left-margin}[Franck JMJM, Han S. “[Overhauser Dynamic Nuclear Polarization for the Study of Hydration Dynamics, Explained]{.nocase}.” In: Wand AJ, editor. Biol NMR part b. Academic Press; 2019. p. 131–175. doi:10.1016/bs.mie.2018.09.024]{.csl-right-inline}
[3. ]{.csl-left-margin}[Franck JM, Scott JA, Han S. “[Nonlinear Scaling of Surface Water Diffusion with Bulk Water Viscosity of Crowded Solutions]{.nocase}.” J Am Chem Soc. 2013 Mar;135(11):4175–4178. PMID: 23347324 doi:10.1021/ja3112912]{.csl-right-inline}
[4. ]{.csl-left-margin}[Hussain S, Franck JM, Han S. “[Transmembrane Protein Activation Refined by Site-Specific Hydration Dynamics]{.nocase}.” Angew Chemie Int Ed. 2013 Feb;52(7):1953–1958. PMID: 23307344 doi:10.1002/anie.201206147]{.csl-right-inline}
[5. ]{.csl-left-margin}[Franck JM, Ding Y, Stone K, Qin PZ, Han S. “Anomalously Rapid Hydration Water Diffusion Dynamics Near DNA Surfaces.” J Am Chem Soc. 2015 Sep;137(37):12013–12023. doi:10.1021/jacs.5b05813]{.csl-right-inline}
[6. ]{.csl-left-margin}[Franck JM, Sokolovski M, Kessler N, Matalon E, Gordon-Grossman M, Han S, Goldfarb D, Horovitz A. “[Probing Water Density and Dynamics in the Chaperonin GroEL Cavity.]{.nocase}” J Am Chem Soc. 2014 Jul;136(26):9396–403. PMID: 24888581 doi:10.1021/ja503501x]{.csl-right-inline}
[7. ]{.csl-left-margin}[Franck JM, Barnes RP, Keller TJ, Kaufmann T, Han S. “[Active cancellation – A means to zero dead-time pulse EPR]{.nocase}.” J Magn Reson. 2015 Dec;261:199–204. doi:10.1016/j.jmr.2015.07.005]{.csl-right-inline}
[8. ]{.csl-left-margin}[Kaufmann T, Keller TJ, Franck JM, Barnes RP, Glaser SJ, Martinis JM, Han S. “[DAC-board based X-band EPR spectrometer with arbitrary waveform control.]{.nocase}” J Magn Reson. 2013 Oct;235:95–108. PMID: 23999530 doi:10.1016/j.jmr.2013.07.015]{.csl-right-inline}
[9. ]{.csl-left-margin}[Demas V, Franck JM, Bouchard LS, Sakellariou D, Meriles CA, Martin R, Prado PJ, Bussandri A, Reimer JA, Pines A. “[‘Ex situ’ magnetic resonance volume imaging]{.nocase}.” Chem Phys Lett. 2009 Jan;467(4-6):398–401. doi:10.1016/j.cplett.2008.11.069]{.csl-right-inline}
[10. ]{.csl-left-margin}[Franck JM, Chandrasekaran S, Dzikovski B, Dunnam CR, Freed JH. “[Focus: Two-dimensional electron-electron double resonance and molecular motions: The challenge of higher frequencies]{.nocase}.” J Chem Phys. 2015;142(21):212302. doi:10.1063/1.4917322]{.csl-right-inline}
[11. ]{.csl-left-margin}[Franck JM, Demas V, Martin RW, Bouchard L-S, Pines A. “[Shimmed matching pulses: Simultaneous control of rf and static gradients for inhomogeneity correction]{.nocase}.” J Chem Phys. 2009 Dec;131(23):234506. PMID: 20025334 doi:10.1063/1.3243850]{.csl-right-inline}
[12. ]{.csl-left-margin}[Beaton AA, Guinness A, Franck JM. “[A modernized view of coherence pathways applied to magnetic resonance experiments in unstable, inhomogeneous fields]{.nocase}.” J Chem Phys. 2022 Nov;157(17):174204. doi:10.1063/5.0105388]{.csl-right-inline}
